Providing REGENERATIVE Services With Compassion
Human Skin Equivalent (Apligraf®)
Apligraf® is an advanced bioengineered skin product designed to treat chronic wounds such as venous leg ulcers (VLUs) and diabetic foot ulcers (DFUs). Apligraf® is a bilayered living cellular construct that mimics human skin, consisting of a dermal layer made of a collagen matrix embedded with neonatal human fibroblasts and an epidermal layer formed by human neonatal epidermal keratinocytes. The application process involves Dr. Brem directly applying Apligraf® to the ulcer after cleaning and preparing the wound bed, allowing the cells to integrate with the patient's own skin and secrete growth factors that enhance wound healing. Dr. Brem successfully uses this product to encourage new tissue formation, reduce inflammation, and accelerate the wound healing process, representing a significant advancement in regenerative medicine by restoring the skin's natural structure and function. Apligraf® has been FDA-approved for over two decades and has shown positive results in numerous clinical trials, transforming chronic ulcers into healing wounds and improving patients' quality of life.
Taub J. Apligraf application for non-healing wound/ulcer [Internet]. YouTube; 2013 [cited 2024 May 28]. Available from: https://www.youtube.com/watch?v=6UaZAP5IB5U
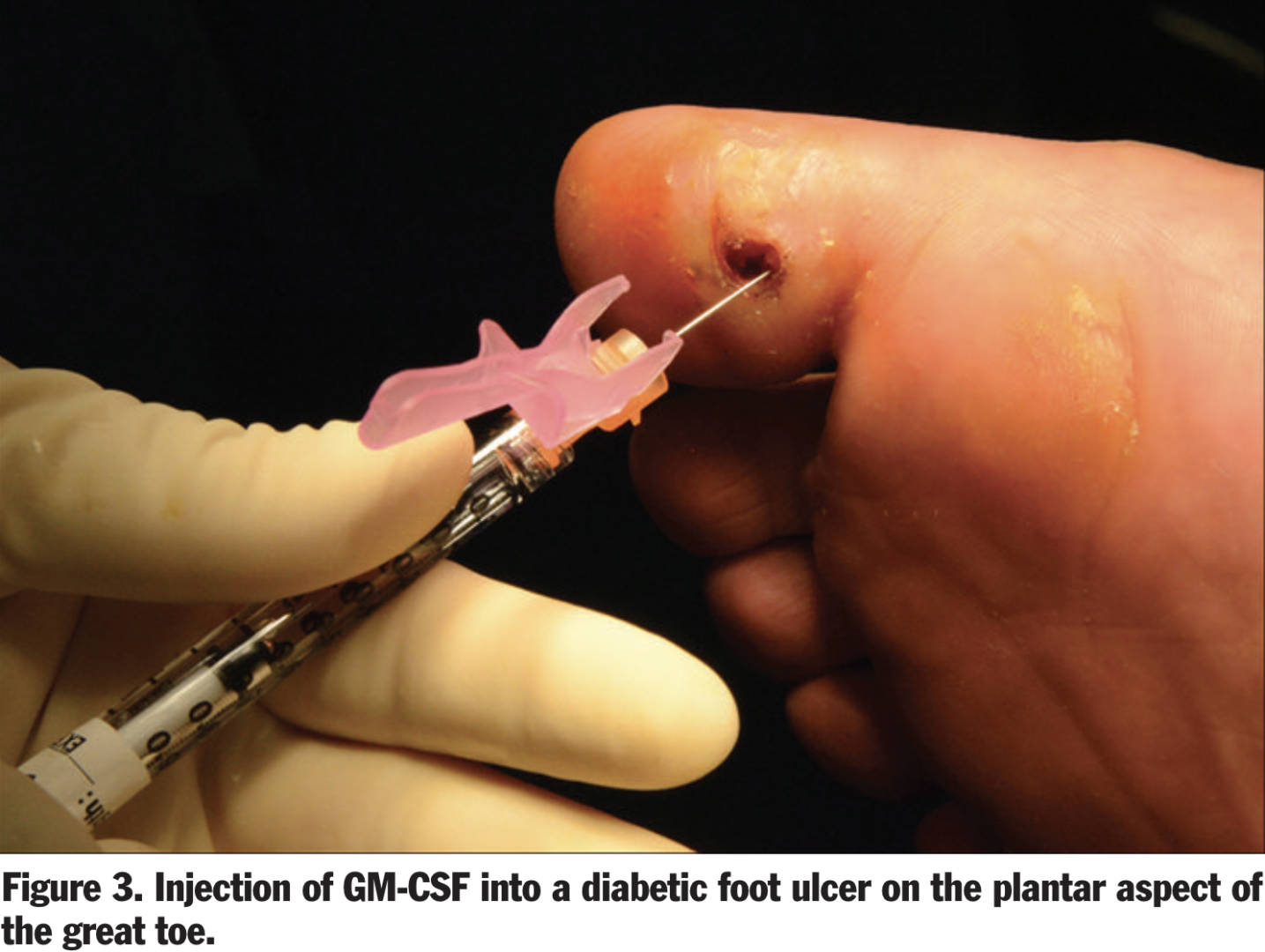
Granulocyte-Macrophage Colony-Stimulating Factor AKA GM-CSF (Leukine ®)
Dr. Brem is advancing wound care by using biologics like granulocyte-macrophage colony-stimulating factor (GM-CSF), which stimulates and recruits cells crucial for healing, such as keratinocytes, macrophages, and fibroblasts. This approach is particularly effective for chronic wounds, which often remain in the inflammatory phase with altered cellular functions and reduced growth factors. Synthetic GM-CSF, administered as a local injection, has shown promise in accelerating chronic wound healing by promoting the proliferation and differentiation of bone marrow-derived monocytes into macrophages, facilitating the transition from inflammation to resolution. Additionally, GM-CSF enhances the production of essential growth factors like VEGF, PDGF, FGF, and TGF-β, aiding processes such as angiogenesis, keratinocyte proliferation, and wound contraction. Dr. Brem's use of GM-CSF in wound care has demonstrated improved healing outcomes, making it a valuable tool in managing complex, non-healing wounds.
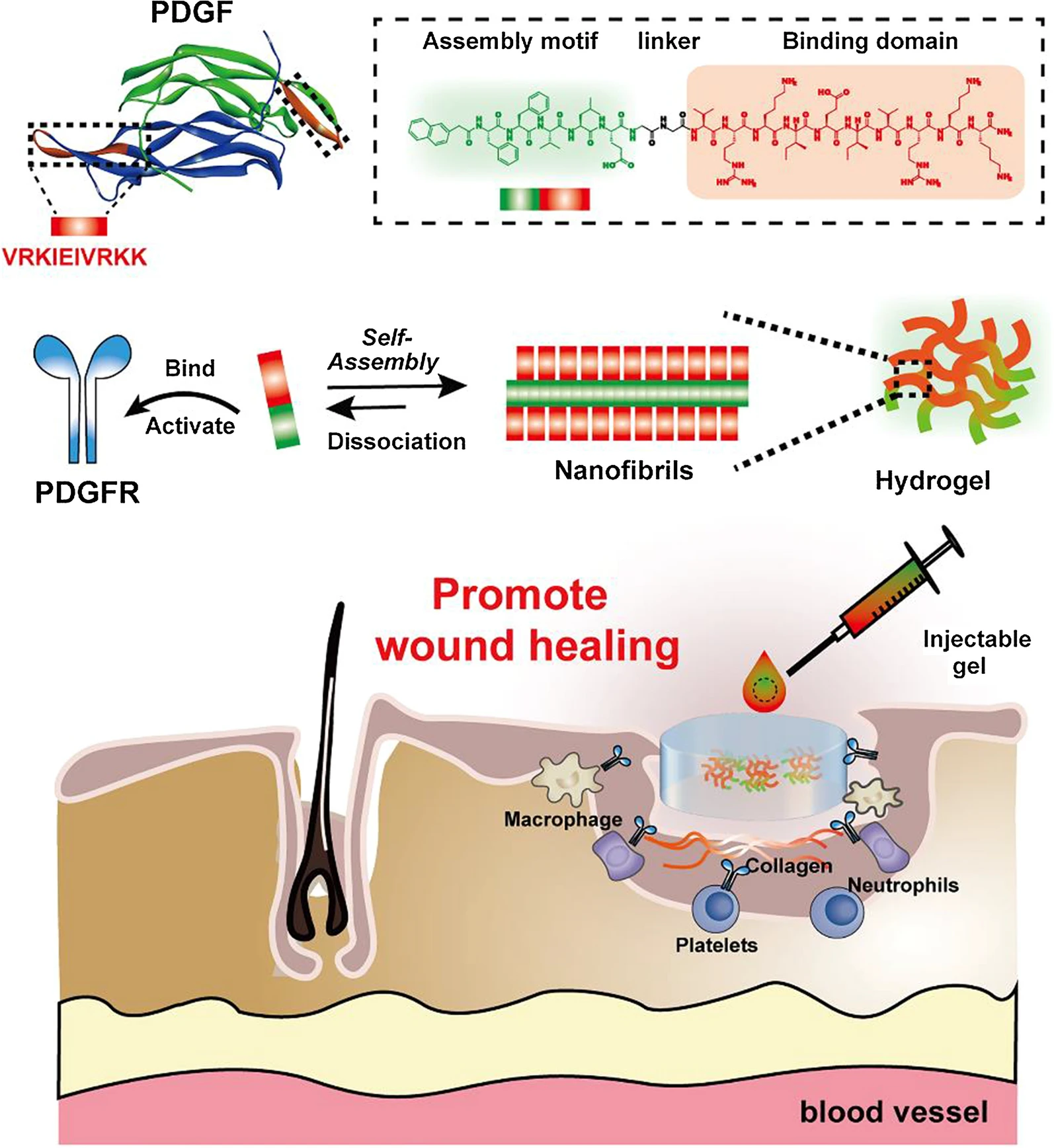
Other Growth Factors: Platelet-derived growth factor-BB (PDGF-BB)
Platelet-derived growth factor (PDGF) is integral to each stage of the wound healing process. PDGF is released from platelets upon injury and is present in wound fluid, playing a crucial role in recruiting neutrophils, macrophages, and fibroblasts to the wound site, thereby initiating the inflammatory response. It also stimulates the proliferation of fibroblasts, which are essential for extracellular matrix production and the formation of granulation tissue. Clinically, recombinant human PDGF (rh-PDGF-BB) has been shown to accelerate wound closure, particularly in diabetic foot ulcers (DFUs). FDA-approved Becaplermin, a topical formulation of rh-PDGF-BB, has significantly increased the incidence of complete wound closure in chronic ulcers, demonstrating its effectiveness in enhancing healing outcomes. Dr. Brem notes that PDGF not only aids in the initial inflammatory phase but also supports the structural integrity of newly formed blood vessels, making it a pivotal factor in both acute and chronic wound healing scenarios.
Other Growth Factors: Vascular Endothelial Growth Factor (VEGF)
Vascular Endothelial Growth Factor (VEGF) is a critical player in the wound healing process, particularly in promoting angiogenesis. Dr. Brem emphasizes VEGF's role in stimulating the formation of new blood vessels, which is essential for delivering oxygen and nutrients to the wound site. VEGF is produced by various cells, including endothelial cells, keratinocytes, and macrophages, and it significantly enhances endothelial cell migration and proliferation. In diabetic and ischemic wounds, VEGF administration has been shown to restore impaired angiogenesis, improving re-epithelialization and overall healing. Intramuscular gene transfer of VEGF and its topical application have demonstrated positive trends in healing rates, making it a valuable tool Dr. Brem uses in the treatment of non-healing wounds.
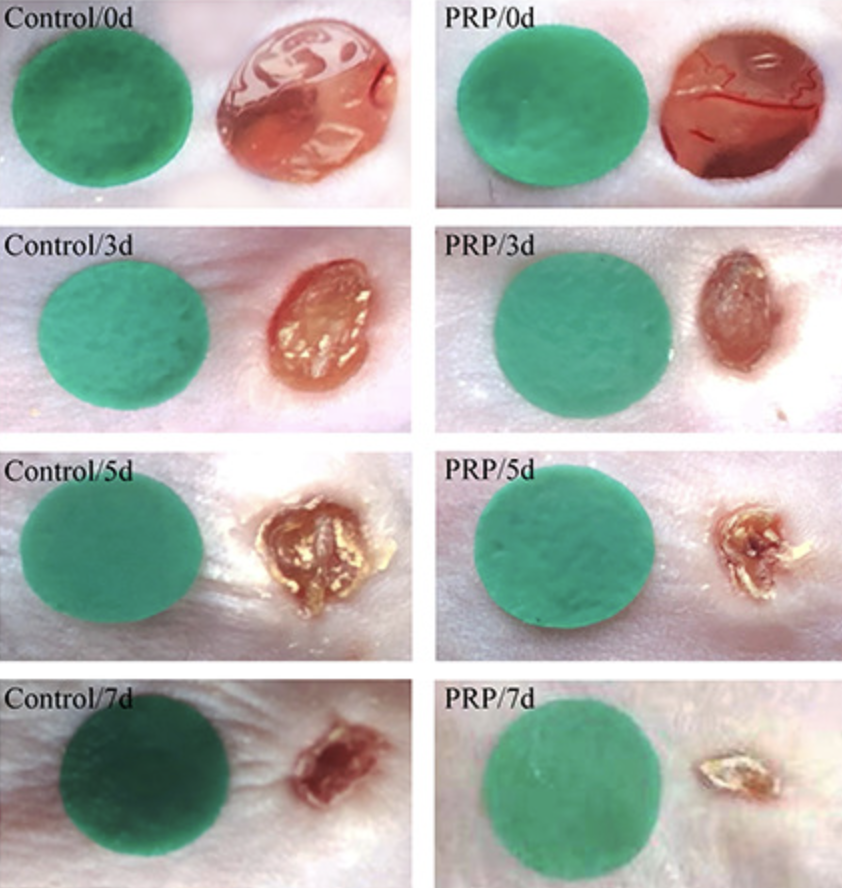
Platelet-Rich Plasma (PRP)
Platelet-Rich Plasma (PRP) is a promising tool in regenerative medicine for wound healing, derived from autologous blood and rich in growth factors like VEGF, IGF-1, and PDGF. These components accelerate wound healing by enhancing re-epithelialization, promoting the proliferation and differentiation of epidermal stem cells (ESCs), and modulating inflammation by reducing pro-inflammatory cytokines such as IL-1β and IL-17. PRP also significantly promotes angiogenesis, ensuring an adequate supply of nutrients and oxygen to the wound site, and aids in wound contraction and collagen deposition, essential for reducing wound size and improving tissue remodeling. This multifaceted approach allows Dr. Brem to use PRP as a valuable adjunct in the treatment of both acute and chronic wounds, offering patients faster recovery, reduced inflammation, and improved overall healing outcomes.
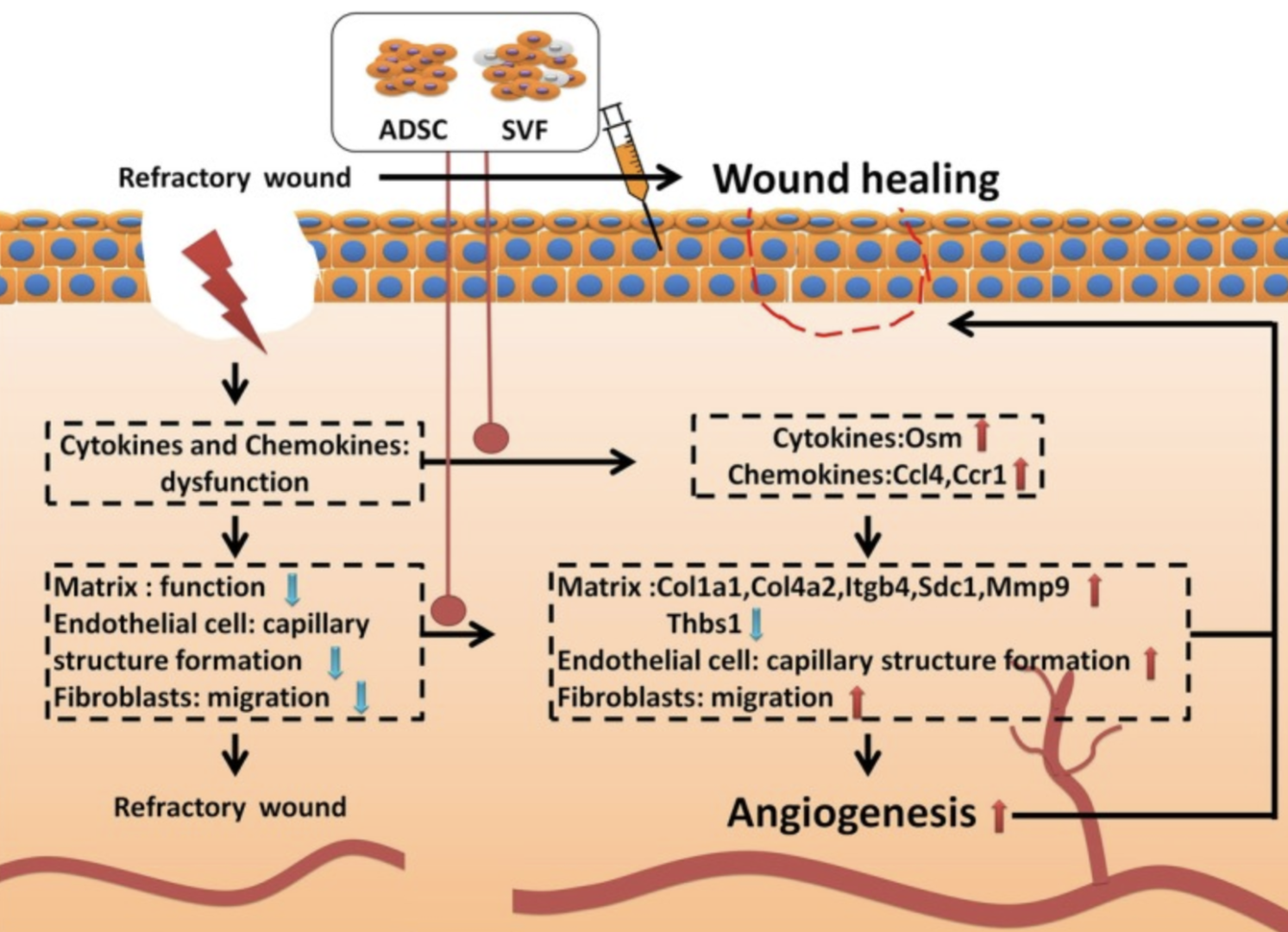
Stromal Vascular Fraction Cells (Stem Cells)
Stromal Vascular Fraction (SVF) cells isolated from mature adipose tissue through digestion, filtration, and centrifugation, consist of a heterogeneous mix of cells, including adipose-derived stem cells (ADSCs). These cells enhance wound healing by promoting angiogenesis, the formation of new blood vessels, and facilitating extracellular matrix (ECM) remodeling. SVF accelerates the migration of fibroblasts and the tubulogenesis of endothelial cells, critical processes for efficient wound repair. By modulating cell adhesion and cytokine pathways, SVF aids in the regeneration of vascular and connective tissues, thereby improving blood supply and nutrient delivery to the wound site. This comprehensive approach, leveraging the innate regenerative capabilities of SVF cells, allows Dr. Brem to significantly benefit patients by accelerating wound closure, reducing inflammation, and enhancing overall healing outcomes.
Extra-Cellular Matrix (ACell®)
Porcine bladder matrix (ACell®) is a promising solution for wound healing, particularly for chronic and non-healing ulcers. Derived from decellularized pig bladder tissue, this matrix retains essential structural proteins like collagen, elastin, and growth factors that support tissue regeneration. The decellularization process removes cellular components to minimize immune rejection, leaving a scaffold that promotes cell attachment, proliferation, and differentiation. When applied to wounds, the matrix recruits the patient's cells, facilitating new tissue formation and accelerating healing. Its inherent growth factors stimulate angiogenesis and collagen deposition, crucial for effective wound healing. Additionally, its resistance to enzymatic degradation ensures sustained support, reducing dressing changes and healthcare costs. Dr. Brem highlights that the porcine bladder matrix actively participates in the healing process by modulating the local wound environment, showcasing its transformative potential in improving patient outcomes and advancing wound care.
Negative Pressure Therapy
Negative Pressure Wound Therapy (NPWT) operates as a regenerative tool that significantly enhances wound healing. By applying controlled negative pressure to the wound bed, NPWT effectively removes excess exudate, reduces edema, and mitigates infection risk. This therapeutic environment promotes the formation of granulation tissue, crucial for wound healing, by enhancing microcirculation and stimulating the production of growth factors. The open-pore foam dressing used in NPWT serves as a scaffold for new tissue growth, supporting cellular proliferation and angiogenesis. Furthermore, the continuous vacuum encourages wound contraction and closure, ultimately accelerating the healing process. Through these regenerative mechanisms, Dr. Brem has transformed the management of complex and chronic wounds, offering patients improved outcomes and faster recovery times.
![WJD-6-1-g002 The 4 primary mechanisms of microdeformational wound therapy: (1) macrodeformation; (2) microdeformation (3) fluid removal; and (4) alteration of the wound environment
Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017; 6(1): 1-16 [DOI: 10.5314/wjd.v6.i1.1]
1. Panayi A, Leavitt T, Orgill D. Evidence based review of negative pressure wound therapy. World Journal of Dermatology. 2017 Jan;6:1–16.](https://newjerseywoundhealing.org/wp-content/uploads/2024/05/WJD-6-1-g002.jpg)